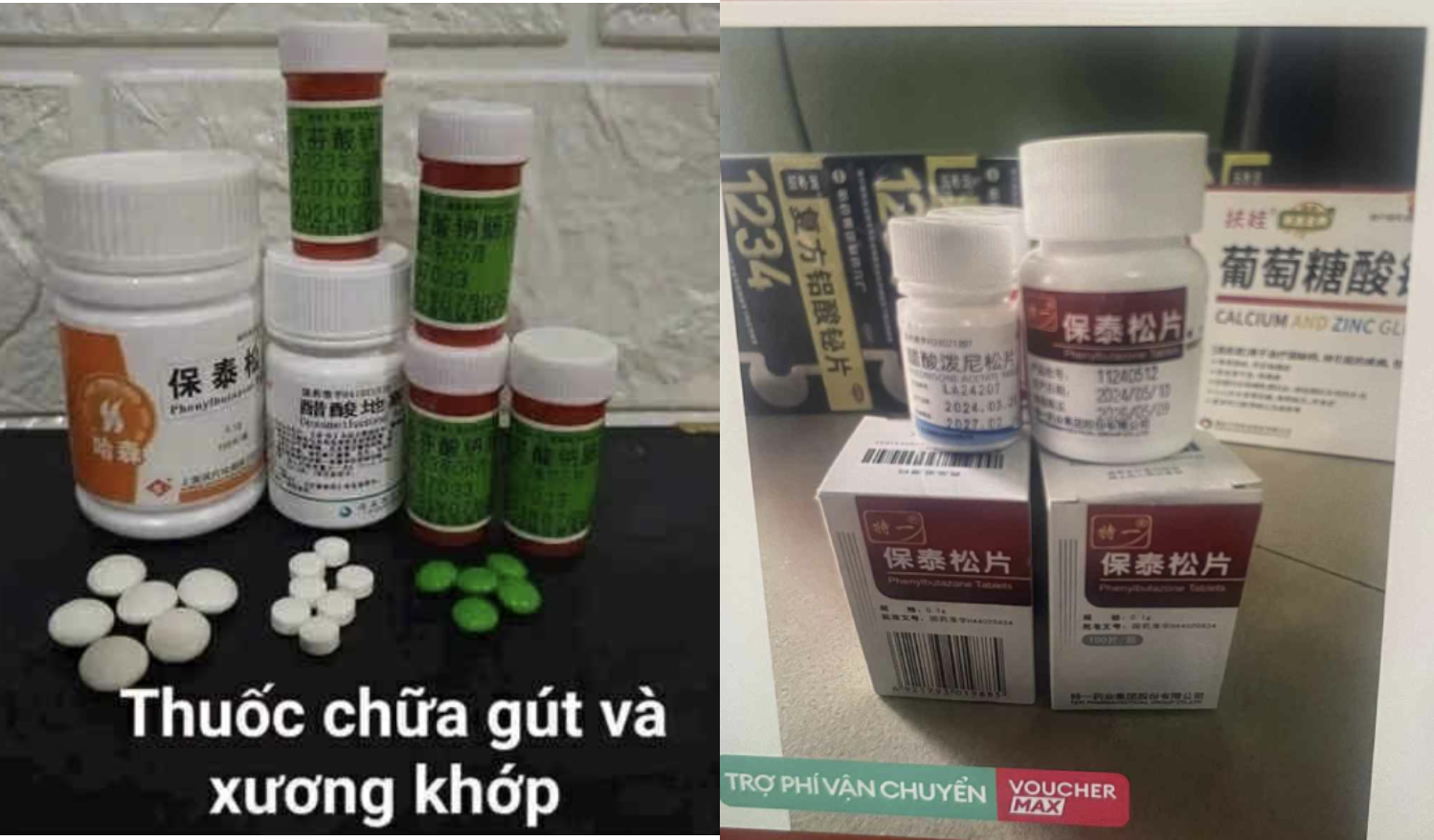
Bach Mai Hospital recently issued an urgent warning after recording several severe allergic reactions caused by Phenylbutazone since the beginning of 2025. The drug, a non-steroidal anti-inflammatory used in the past to treat acute gout and ankylosing spondylitis, has been banned by the Ministry of Health since 2001.
Among the affected patients, one person died and three are in critical condition, all of whom had self-medicated. The cases include three men and one woman, with the youngest patient only 39 years old. The fatality was a 70-year-old woman from Phu Tho.
Despite the ban, VietNamNet has found that drugs containing Phenylbutazone are still being sold openly on e-commerce platforms like Shopee and Lazada, advertised as treatments for arthritis and acute gout. Prices range from 170,000 to 220,000 VND (approximately 7 to 9 USD) per box depending on the seller.
Some accounts even claim the products are hand-carried from China, not classified as banned, and safe for use.
Addressing the issue, Ta Manh Hung, Deputy Director of the Drug Administration of Vietnam, stated on August 19 that the Ministry of Health has not issued any valid circulation licenses for drugs containing Phenylbutazone.
"The Drug Administration has not licensed the importation of Phenylbutazone as a raw material or as a finished drug product," Mr. Hung affirmed.
Dr. Chu Chi Hieu, Head of the Allergen Department at the Center for Allergy and Clinical Immunology, Bach Mai Hospital, explained that Phenylbutazone is banned due to its high risk of causing severe allergic reactions, multi-organ failure, and even death.
To protect public health, the Drug Administration has requested health departments in 34 provinces and cities to notify all pharmaceutical businesses, healthcare providers, and citizens not to purchase, sell, or use any drugs containing Phenylbutazone or any unlicensed, unknown-origin medicines.
"If any product containing Phenylbutazone is discovered on the market, it must be reported immediately to the local health authority and relevant agencies for timely inspection and enforcement," the Drug Administration emphasized.
Health departments are also urged to work with relevant agencies, including the 389 Anti-Smuggling Steering Committee, police, and market surveillance forces, to enhance monitoring and crack down on the sale and promotion of unlicensed medicines, especially those being sold or advertised online in violation of regulations.
For licensed pharmacies and healthcare institutions with websites, the Drug Administration has instructed them to review all posted content and remove any misleading advertisements about Phenylbutazone products that could wrongly imply they are approved for use in Vietnam.
Vo Thu