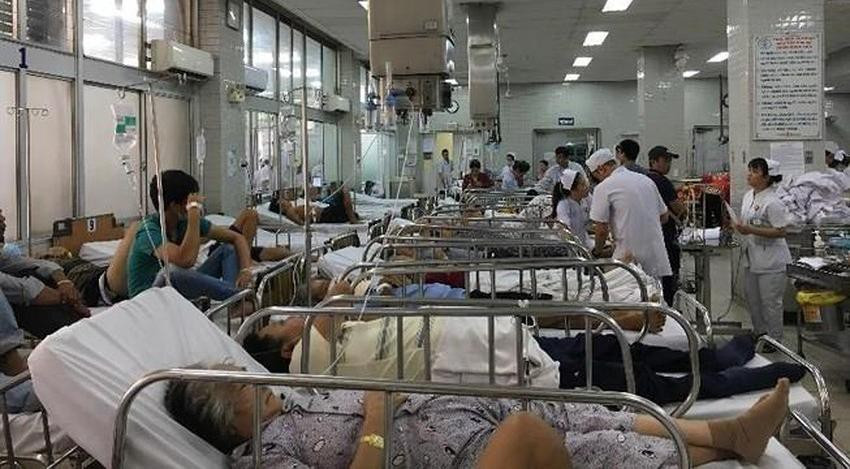
Some public hospitals in Hanoi and HCM City have complained about a lack of medicine. Hanoi-based Bach Mai, one of the country’s largest hospitals, has reported a shortage of antidotes, while the Central Hospital of Odonto-Stomatology has complained about a lack of anesthetics, used by more than 70 percent of outpatient services.
The medicine shortage is blamed on tardiness in extending drug registration and the Covid-19 pandemic over the last two years.
The problems in bidding procedures and several scandals in the healthcare sector in which people have been prosecuted have also caused hospital leaders to balk at making procurement and bidding plans.
Law on Pharmacy amendments
Deputy Minister of Health Do Xuan Tuyen believes that amending the 2016 Law on Pharmacy, which has shown shortcomings, is a necessity to clear congestion in bidding for medicine for public hospitals.
He said certificates on GMP (Good Manufacturing Practice) and drug registration certificates need to be automatically extended to be in line with international practice . Otherwise, enterprises will face difficulties in material imports, which will cause interruption to the production, circulation and supply of drugs.
Noting that 10,000 drug registration certificates will expire by December 31, 2022, Tuyen said if regulations on automatic extension are not amended, the drug shortage will be unavoidable.
At the September 20 session, the National Assembly Standing Committee discussed the Law on Bidding amendments, pointing out difficulties in organizing the bids for drug and medical equipment supply. It said there was a need to amend the Law on Bidding to be sure that the healthcare sector can procure medical equipment and drugs.
National Assembly Chair Vuong Dinh Hue asked to clarify some issues in drug bidding, especially the regulation that "drug prices this year must be lower than the previous year". He questioned if the regulation is suitable if demand for healthcare services increases.
Three months ago, President Nguyen Xuan Phuc mentioned a "crisis in the healthcare sector". At a working session with the healthcare sector and relevant ministries, Prime Minister Pham Minh Chinh concluded that "hospitals should buy medicine at reasonable prices, not the lowest prices".
Most recently, at the meeting of the National Steering Committee on Covid-19 Prevention and Control on September 13, 2022, Prime Minister Chinh once again said the lack of drugs, bioproducts and medical equipment must not persist for long periods, often caused by administrative procedures and irresponsibility.
“Those who don’t want to do it, please stand aside,” he said, adding that tardiness in drug procurement will not protect people’s lives and health.
Ensuring the right to access brand-name drugs
In Vietnam, after the regulations on drug bidding were issued, the bidding of brand-name drugs, mainly those with the highest quality, safety and effectiveness, was mentioned regularly.
This shows that doctors’ and patients’ have a right to access brand-name drugs in accordance with the Law on Medical Examination and Treatment.
According to IQVIA, brand-name drugs account for 11 percent of total prescription drugs used (the figure is 27.1 percent on average in Asia Pacific).
Currently, Vietnam’s factory prices of brand-name drugs are among the lowest in ASEAN and they continue to fall through price negotiations. Nearly two-thirds of brand-name drugs now in use have expired copyright protection.
In general, brand-name drugs cannot be replaced when the copyright protection expires. Generic drugs are just bio-equivalent. Therefore, if drugs are bid together with Group 1 - generic drugs, they won’t be competitive in price.
So, it is difficult for brand-name drugs to be available at clinics and drugstores. As a result, patients cannot access good drugs, while doctors don’t have the opportunity to upgrade their knowledge about modern medicine, according to Pham Khanh Phong Lan, a leading expert in healthcare policies.
This leads to a risk of brand-name drug smuggling, which is difficult to control.
Under current regulations, brand-name drugs are present in medical facilities’ list of drug procurement. However, the Ministry of Health (MOH) is still amending regulations and it is unclear whether it will maintain brand-name drug bidding packages.
Van Thieng