 |
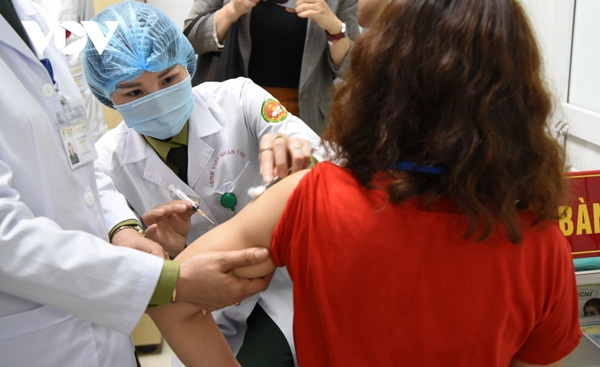
| Volunteers participate in the clinical trials of the Nanocovax vaccine. |
According to the preliminary evaluation of the National Ethics Committee in Biomedical Research under the Ministry of Health, the domestically-developed vaccine fully meets all safety and immunogenicity requirements.
Moving forward, researchers must submit additional documents early next week in order for the Ministry to review before releasing the final press release.
The clinical trials of phase 3a involved the participation of 1,004 volunteers in both Hanoi and Long An.
The volunteers received their initial jab from June 8, with the second shot being given after 28 days, with the monitoring period scheduled to end in early June, 2022.
The selection of samples of volunteers participating in phase 3a has been done to ensure gender balance and diversity in age groups, with the youngest participant being 18 years old and the oldest 81.
Furthermore, researchers also allowed volunteers with a history of allergies, chronic heart disease, diabetes, hypertension, cancer, and obesity to participate in the clinical trials.
The results of the phase 3a indicate that all participants recorded very good immunogenicity results, with no serious side effects. In addition, the Nano Covax vaccine had a seroconversion rate of up to 99.2% at the 42nd day.
Moreover, the assessment results of the rate of virus neutralising antibody titers by PRNT50 method for those who have been injected reached 96.5%.
With the Nano Covax vaccine therefore meeting the requirements for immunogenicity, the research team has moved to recommend that phase 3b can be conducted on 12,000 volunteers.
Nano Covax represents the first domestically-developed COVID-19 vaccine and is being produced by Nanogen Pharmaceutical Biotechnology JSC based on recombinant DNA/protein technology.
Source: VOV

S.Korean firm to provide Vietnam’s Nano Covax vaccine worldwide
Korean pharmaceutical firm HLB has signed an MoU with Nanogen, producer of Vietnam's Nanocovax Covid-19 vaccine, to acquire the rights to distribute the vaccine worldwide, except in Vietnam and India, when the vaccine is approved for use.