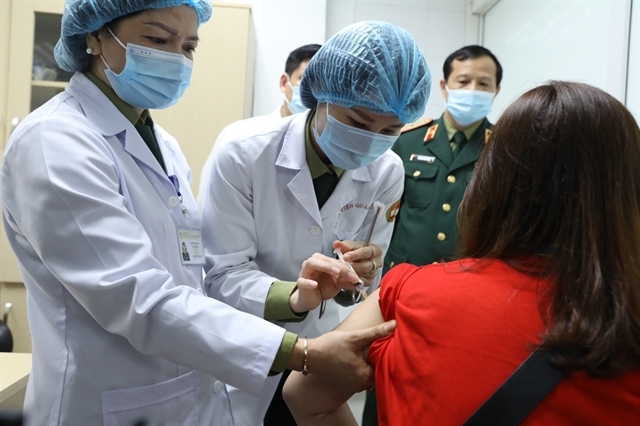
Nanocovax is Vietnam’s first COVID-19 vaccine reaching the human trial stage, developed by Nanogen Pharmaceutical Biotechnology JSC and the Military Medical University (MMU) in Hà Nội.
According to a representative from MMU, the phase 2 was held concurrently at two places, 35 were given the jabs at the MMU and Pasteur Institute in HCM City will administer the shots to 39 volunteers in the southern province of Long An.
The Phase 2 trial is expected to see the participation of up to 560 volunteers aged 18-60. Some of them have underlying health conditions such as hypertension and diabetes but are not too severe.
The trial of the vaccine at the two places, compared to the first phase where the vaccine is being administered in Hanoionly, could speed up the progress by shortening the study time to three months instead of six months while ensuring scientific data is accurate, the representative said.
The first 35 volunteers in Hanoiwere chosen among about 300 people who registered to participate in the second-stage trial of the vaccine at the MMU after undergoing rigorous medical examinations.
They are divided into four random groups to receive three doses of 25mcg, 50mcg and 75mcg of the vaccine or a placebo.
“Because the trial sees the participation of volunteers with underlying health conditions, we have been prepared for all scenarios and ensured safety for all volunteers,” said Lieutenant-General Do Quyet, Director of the Military Medical University.
He said the results of the trial would be announced in May before the third stage trial during which only one single shot of the vaccine is to be injected for 10,000-15,000 people from domestic and foreign pandemic-hit areas.
Test results of the first phase of the COVID-19 vaccine showed that it is safe and has no serious complications and might be effective against B117, the new variant in the United Kingdom, Quyet said.
He also said the research team would evaluate the immune response carefully in the second phase.
“Although we have shortened the trial of the vaccine by half with great efforts by the research units, more efforts should be made and the compliance with set standards is a must,” said Deputy Prime Minister Vu Duc Damas he visited the first 35 volunteers on Friday.
He stressed the need to have a COVID-19 vaccine produced by Vietnam as quickly as possible, adding that the successes of the trial would not only serve as effective against COVID-19 but also boost the pride of Vietnamese scientists who take part in the research and development of vaccines as well as of the public at large.
At the vaccine administration site in the southern province of Long An, a 57-year-old man from Thanh Phú Commune in the province’s Bến Lức District told Vietnam News: “After I heard about the second phase of human trials on the media, I went to the health centre to register as a volunteer.”
“I feel very happy to get the vaccine.”
Another volunteer, 51, from the same commune said: “I was provided careful counselling about the vaccine. I also read about the results of the first phase. I trust the vaccine’s effectiveness. I was happy to be selected.”
Nguyen Ngo Quang, deputy director of the Department of Science, Technology and Training, said: “Besides importing COVID-19 vaccines, the country also has to research and develop the vaccine to ensure health security.”
The imported vaccines are only meant for use now, and the country has to rely on domestic production in future, according to Quang.
The country also has other vaccine candidates being developed by the Institute of Vaccines and Medical Biologicals, the Company for Vaccine and Biological Production No 1 and the Centre for Research and Production of Vaccines and Biologicals.
VNS

Government issues resolution on COVID-19 vaccine purchase, use
The Government on February 26 issued Resolution No 21/NQ-CP regarding COVID-19 vaccine purchase and use.