 |
|
|
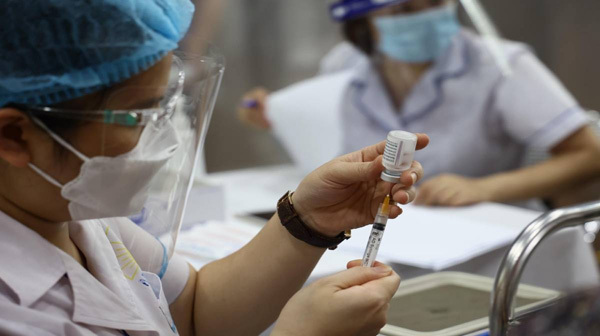
The clinical trial for the ARCT-154 vaccine has been approved by the Ministry of Health. Hanoi Medical University, Ho Chi Minh City Pasteur Institute, and Military Medical Academy are responsible for the trial.
Phase 1 trials with 100 volunteers will start on August 15, conducted at Hanoi Medical University.
The university is seeking volunteers, aged 15-59 years, with no history of SARS, MERS or SARS-CoV-2 infections for the trial. Volunteers must have never been vaccinated against Covid-19; and agree to follow the study's procedures and take at least eight clinical examinations according to the study schedule at the Center for Clinical Pharmacology of the Hanoi Medical University.
Over 300 volunteers will be involved in phase 2 trial. Phase 3 will be conducted with 20,600 volunteers, including 600 volunteers in phase 3a and 20,000 volunteers in 3b phase.
The ARCT-154 vaccine uses the latest mRNA vaccine technology able to fight the Delta variant. The vaccine is produced by Arcturus Therapeutics, Inc., USA. A private group of Vietnam received the technology transfer from the US producer.
This is the third Covid-19 vaccine in Vietnam in clinical trials, after Nanocovax of Nanogen and Covivac of IVAC.
Thuy Hanh
Vietnam consults experts on developing homegrown COVID-19 vaccines
The Ministry of Health in collaboration with the World Health Organisation organized a webinar on August 4 to consult experts on the evaluation of clinical research data and approval of vaccines against COVID-19 in emergency situations.