The customs has explained the discard of 20,000 pills for cancer treatment over expiration is not due to its lengthy import procedures, says a press release which the Ministry of Finance issued on May 8.
In fact, for humanitarian reasons, the customs office in charge has quickly cleared the shipment one day after a customs declaration was filed. It has complied with the regulations and the opinions of the relevant management agency, namely the Drug Administration of Vietnam, on the remaining shelf life of the drug when it arrived at Vietnam port.
On the import drug management policy, the customs says drugs imported into Vietnam must have circulation registration certificates and import permits that are still valid.
In addition, they must meet the rules on drug shelf life given in Circular 45/2011/TT-BYT dated December 21, 2011 and Clause 3, Article 2 of Decision 42/QD-TTg dated July 15, 2013 on donated drugs, humanitarian aid, rare drugs, and drugs for treatment needs of hospitals.
The drugs whose duration is 24 months or more should have a remaining shelf life of at least 12 months from the date of arrival in Vietnam. In case the drugs are fit for use in less than 24 months, their remaining duration from the date of arrival at a local port must be at least equal to one third of their shelf life.
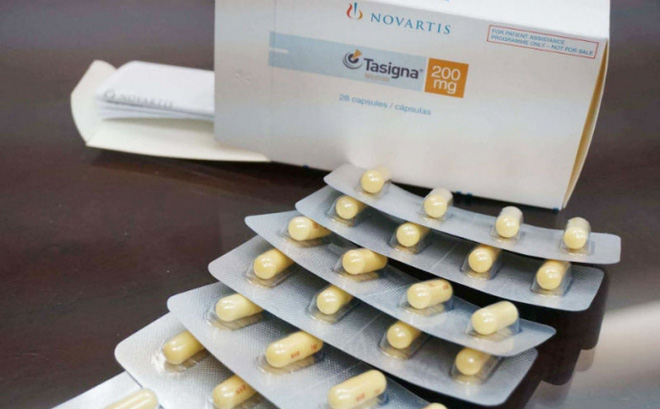
The media has recently reported on the destruction of 20,000 cancer pills. This drug batch is manufactured by Novartis Pharma AG with a 24-month duration (production date: June 2013, expiry date: May 2015).
In the press release, the customs states that all the prolonged procedures are due to other agencies, not the customs red tape as assumed by the public.
As per the explanation, on July 15, 2013, the HCMC Hospital of Hematology and Blood Transfusion received a letter on drug donation. On November 28, 2013, the hospital sent Official Letter 1639/TMHH-KHTH to the Drug Administration of Vietnam seeking permission to receive the donation from Novartis Pharma AG.
Two weeks later, the drug administration wrote to the hospital stating its disapproval due to a lack of necessary papers.
Then, on March 10, 2014, or three months later, the HCMC Department of Health wrote to the municipal government asking for permission to receive the aid. It was not until June 24, 2014 that the HCMC government gave the nod for the health department to receive the drug.
On July 14, 2014, the Drug Administration of Vietnam issued another letter allowing the HCMC Hospital of Hematology and Blood Transfusion to receive the shipment. It was clearly stated that the remaining shelf life of this shipment shall not be less than 12 months from the date of arrival in Vietnam.
According to Bills of Lading No. 740135202-2 and 7401351230, the batch was loaded onto an aircraft for transportation to Vietnam on July 23, 2014. Thus, at the time of its arrival at a Vietnamese port, the remaining duration was under 12 months since the production date is June 2013).
On August 1, 2014, the blood transfusion hospital wrote to the HCMC Department of Customs explaining why the remaining shelf life of this shipment was under 12 months and asking for clearance due to humanitarian reasons. Five days later, express delivery company DHL-VNPT on behalf of the hospital made two customs declarations 051586/PMD and 051587/PMD. The courier express division of the HCMC Department of Customs carried out the procedures immediately for the above two declarations, both of which were cleared on August 7, 2014.
Given the timelines stated in the letter of explanation, it took more than one year since the donation was announced until the drug shipment reached Vietnam, while it took only a few days to accomplish customs clearance for the batch.
SGT