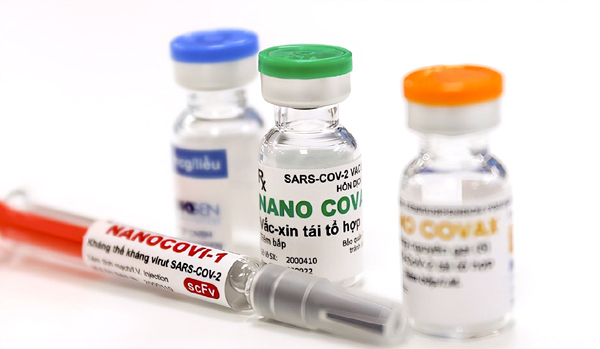
Nanocovax developed by Nanogen is in the third phase of a clinical trial. It has been given to 13,000 volunteers, and the final shots are being implemented before the preliminary report is released in late August or early September.
According to Nanogen, the publishing of experimental data in scientific journals will help increase the reliability of the research, and open the research up for opinions and comments from scientists around the world.
To ensure transparency in research, Nanogen’s scientists published two articles on two open-data science portals – BioRxiv (July 20) on preclinical results, and clinical results phases 1-2 on MedRxiv (July 22).
In an article published in MedRxiv, Nanogen’s scientists mentioned the safety and immunogenicity of the Nanocovax vaccine.
Nanogen uses a recombinant protein method to produce the Nanocovax vaccine.
All volunteers had to undergo screening, have clinical and biological examinations and have tests (complete blood count, biochemistry, urinalysis, pregnancy testing, and imaging studies). Those who had had Covid-19 or tested positive for SARS-CoV-2 were not eligible for the trial.
In the first phase, from December 17, 2020, 60 volunteers aged 18-50 got two shots to assess the safety of the vaccine (the time between the two shots was 28 days).
In the first phase, no serious side effects were discovered. Most of the side effects were level 1 and ended after the injection.
In the second phase, 480 volunteers received vaccines at three dose levels, while the other 80 were injected with a placebo.
The healthy volunteers with BMI from 17-35 were classified into three groups 18-45 years old, 46-60 and over 60. The oldest volunteer was 76.
The side effects were mild and they finished within three days . There was no serious side effect related to the vaccines.
After the first and second phases, the research team could prove that Nanocovax has high safety. Most side effects and level-1 serious effects ended after 48 hours.
The research team concluded that Nanocovax vaccine is well tolerated and produces a strong immune responses. The team wants to select a 25 mcg dose for the phase 3 trial to assess the effectiveness of the vaccine.
However, the research team admitted some problems in the research phase 1 and 2, which included the lack of ethnic diversity. The trials were implemented only in Vietnam, and the supervision period was short. And finally, the trial was implemented only on healthy volunteers.
Nguyen Hung
HCM City needs 5.5 million Covid-19 vaccine doses in August
Ho Chi Minh City, the country's current largest COVID-19 hotspot, is estimated to need 5.5 million doses of vaccine in August, according to the municipal People’s Committee’s proposal on vaccine distribution.