- © Copyright of Vietnamnet Global.
- Tel: 024 3772 7988 Fax: (024) 37722734
- Email: evnn@vietnamnet.vn
nanocovax
Update news nanocovax
Are Vietnam’s locally made Covid-19 vaccines viable?
Among the vaccines developed by Vietnamese companies, NanoCovax is considered the one that can ‘go far’, though the end point is unclear.
National Ethics Committee approves phase 3 study of homemade Covid-19 vaccine
In the evening of September 18, the National Ethics Committee in Biomedical Research under the Ministry of Health (MoH) approved the clinical trial results of the phase 3 of Nanocovax, a home-grown Covid-19 vaccine.
S.Korean firm to provide Vietnam’s Nano Covax vaccine worldwide
Korean pharmaceutical firm HLB has signed an MoU with Nanogen, producer of Vietnam's Nanocovax Covid-19 vaccine, to acquire the rights to distribute the vaccine worldwide, except in Vietnam and India, when the vaccine is approved for use.
Procedures to be simplified for emergency licence for local Nanocovax vaccine
The Prime Minister has assigned the Ministry of Health to shorten administrative procedures for granting an emergency licence for the locally made Nanocovax vaccine against Covid-19.
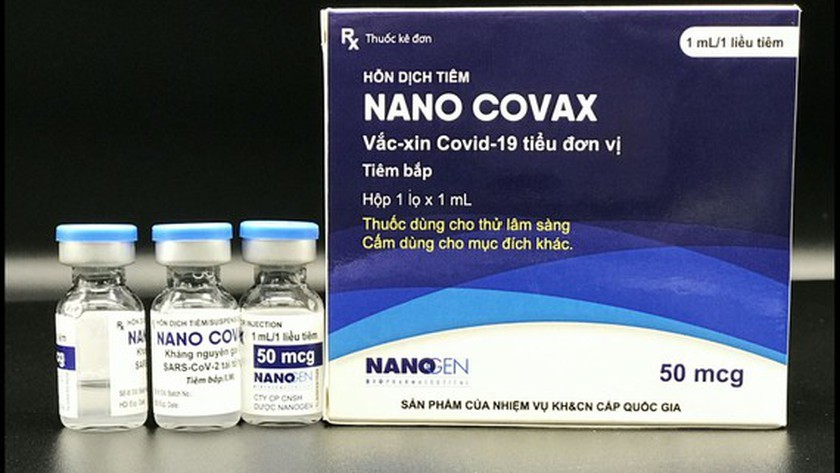
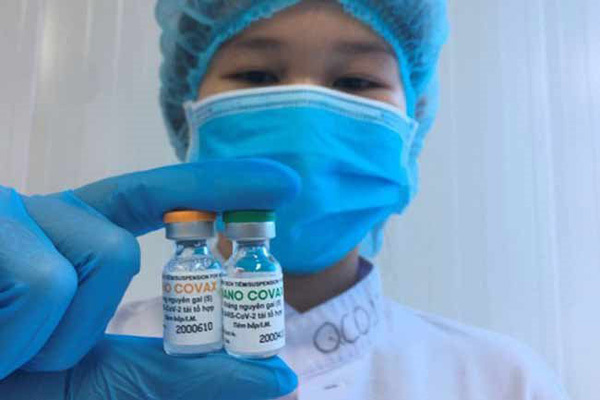
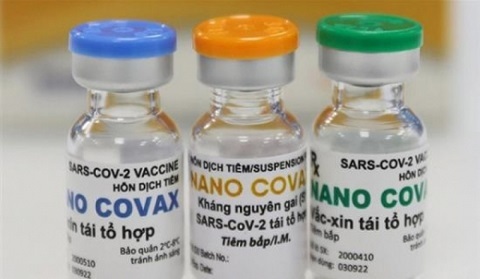
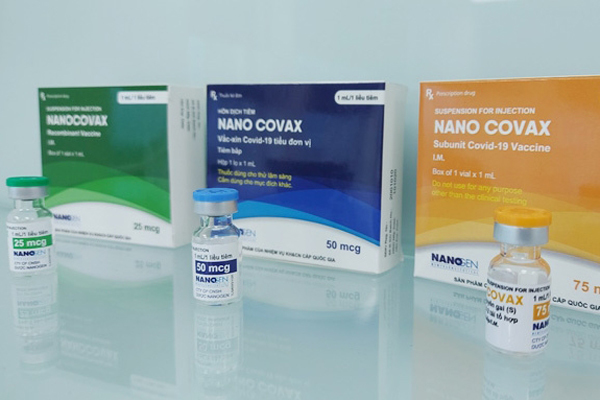
Vietnam’s Nano Covax is 90% effective against SARS-CoV-2
Nano Covax is Vietnam’s first COVID-19 vaccine that is entering the third phase of human clinical trial with the participation of 13,000 volunteers. All the volunteers are set to receive two shots by August 15.
Results of research on Nanocovax vaccine published in open-data science portal
Nanogen, a Vietnamese biotechnology and pharmacy firm, has published two scientific articles on the results of research on the Nanocovax vaccine.
Three provinces want to pilot locally-made vaccine Nanocovax
At least three provinces, including Binh Duong, Khanh Hoa and Dong Thap, said they want to participate in the trial phase of the locally-made Covid-19 vaccine Nanocovax.
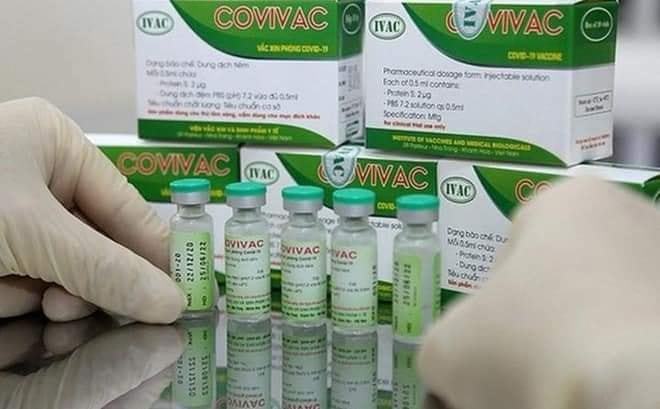
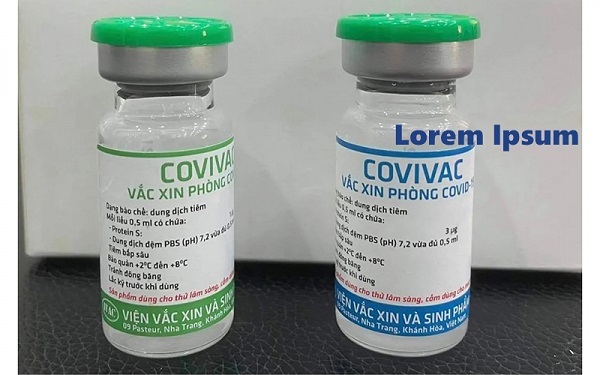
Locally made COVID-19 vaccine Covivac evaluated in Canada
Samples of Covivac, a Vietnamese produced coronavirus vaccine, have been sent to Canada for evaluation of its effectiveness, according to Prof. Dr. Dang Duc Anh, director of the National Institute of Hygiene and Epidemiology (NIHE).
Health Ministry urges speeding up of locally made Covid-19 vaccines
After an emergency meeting, the National Ethics Council agreed to test the locally-made Covid-19 vaccine Nanocovax on 13,000 volunteers. This third phase trial will be completed in mid-August.
Made-in-Vietnam Nanocovax is expected to be as good as foreign vaccines
The test of locally-made Nanocovax vaccine has yielded positive signs. In case of emergency approval, Vietnam would be able to produce 100 million doses per year.
Trials of homegrown COVID-19 vaccine show positive signs
The clinical trials of Vietnamese COVID-19 vaccine candidate Nano Covax are set to enter Phase 3 this month with the optimal dosage of 25mcg, aiming to further test the safety and efficacy of the vaccine in humans before mass production begins.
Local Nanocovax vaccine's phase 3 trial to begin next week
Next week, Vietnam will start the phase 3 trial of the Vietnamese-made Nanocovax vaccine on about 13,000 volunteers.
Vietnam to have its first Covid-19 vaccine
Results of a clinical trial phase 2 of made-in-Vietnam Covid-19 vaccine Nanocovax showed that 100% of vaccinated people made antibodies to fight the virus. The vaccine was also effective on the British variant of the coronavirus.
Vietnam to soon produce COVID-19 vaccine
The time for research and production of Nano Covax, Vietnam’s first candidate vaccine to reach human trial stage, has been shortened as much as possible, but safety has been ensured, said a health official.
Vietnam to produce Covid-19 vaccines by September
The clinical trial of the domestically-made Nanocovax vaccine is going smoothly, and by the end of September, Vietnam will have its first Covid-19 vaccine.
Initial phase of clinical trials on Covivac begins on March 15
The Hanoi Medical University is to start the initial phase of clinical trials on the second domestically-produced COVID-19 vaccine known as Covivac, developed by the Institute of Vaccines and Medical Biologicals (IVAC) on March 15.
Vietnam's Covid-19 vaccine completes 50% of phase 2 trial
Vietnam has completed the first-dose vaccination of 560 volunteers who participated in the Phase 2 trial of Nanocovax's Covid-19 vaccine,
Vietnam's aims to have 150 million doses of Covid-19 vaccine
While negotiating with foreign vaccine providers, Vietnam will accelerate vaccine purchases to ensure that all people have access to Covid-19 vaccines.
Second phase of Nano Covax human trials to begin this month
The second phase of the human trials of Nano Covax, a homegrown COVID-19 vaccine, will begin on February 26, following the first phase that has been proven safe.
Vietnam completes first phase of Nano Covax human trials
Vietnam has completed the first phase of the human trials of Nano Covax, a homegrown COVID-19 vaccine, with the participation of 60 volunteers, the Vietnam Military Medical University said on February 8.